-

50+ Radiopharmaceuticals Automated Synthesis
-

Compliant with GMP Production Standards
-

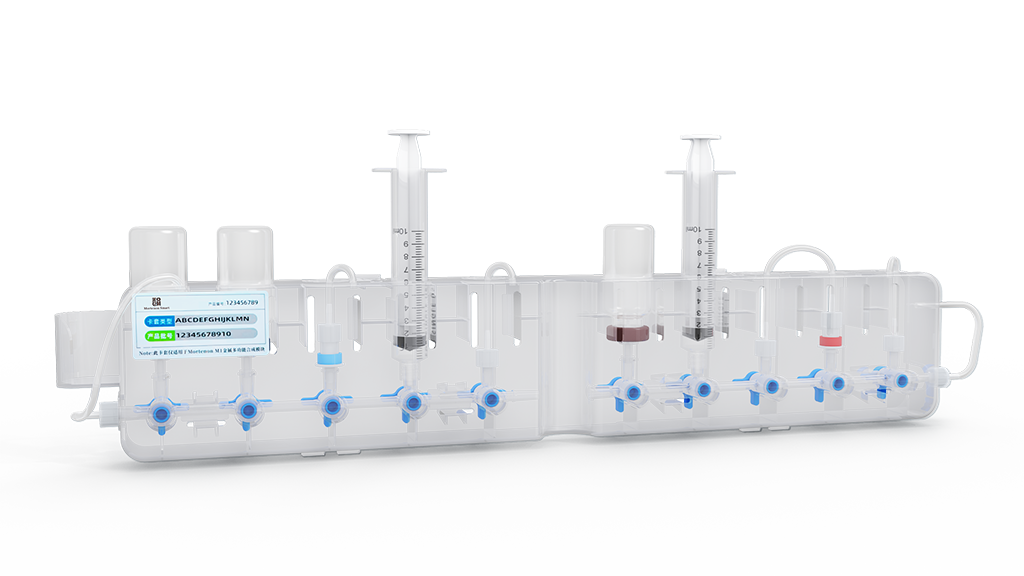
"Cassette-Tape" Integrated Cartridge
-
Intelligent Control Software
Product Details
-
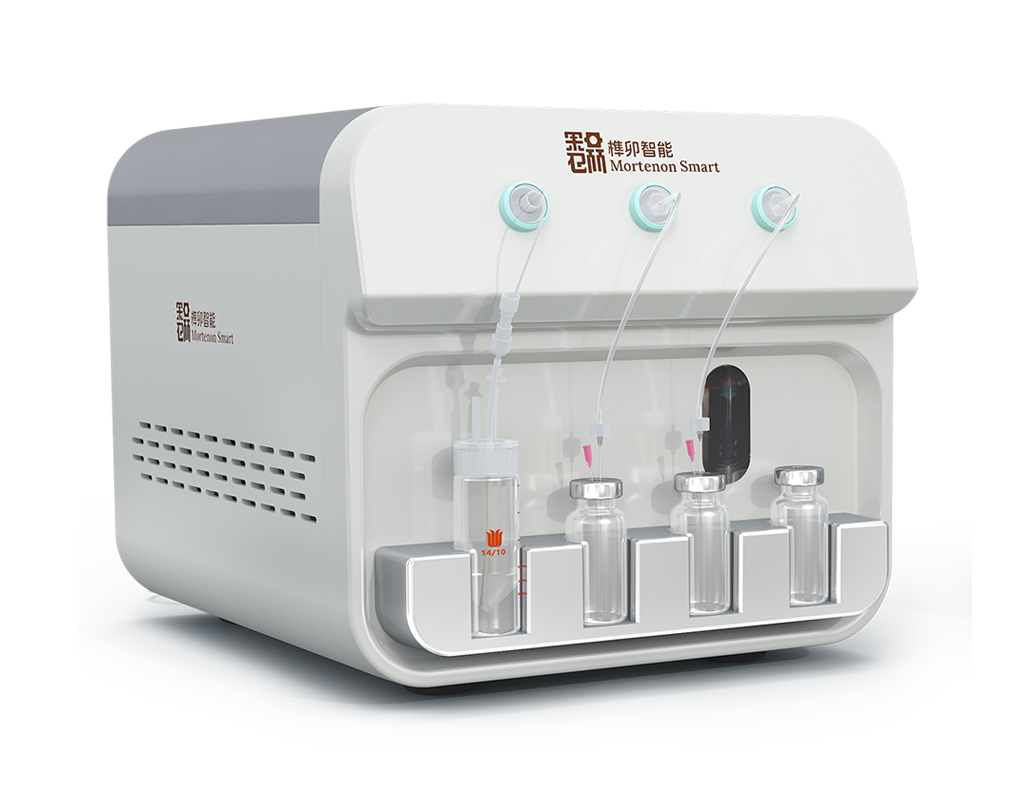
Modular Combination Design
The main unit can be flexibly paired with a variety of functional components …Its compact design supports on-demand configurations, and can adapting to various on-site environment and installation methods.
-
User-Friendly
Integrated "Cassette-Tape" Type Cartridge Design, eliminating the need for complex tubing connections and inspections, enables quick installation and removal. Paired with a user-friendly operating software, it is simple and easy to operate.
-
Safety Protection
The liquid pathway in the cartridge achieves 100% isolation from the main unit. After cartridge removal, the main unit retains no radioactive residue, making it suitable for labeling with long-half-life radionuclides.
-
Powerful Functionality
The operating software is pre-loaded with radiolabeling protocols for nuclides such as ⁶⁸Ga, ⁸⁹Zr, ⁶⁴Cu, ¹⁷⁷Lu, ¹⁸F, ⁹⁹mTc, ¹³¹I; Includes dozens of mature tracer synthesis protocols for direct use.
Product Portfolio




Product Videos
Customer Reviews
Q&A
-
Can the Mortenon M1 accommodate customized workflows from partners?The Mortenon M1 was designed from the outset with a strong emphasis on scalability and compatibility. We support co-development with partners to create customized cassettes and synthesis protocols, ensuring the system maintains high technical adaptability and forward compatibility throughout product iteration cycles. In other words, the Mortenon M1 is not just an automated device — it is a future-ready, continuously evolving platform for radiopharmaceutical production.
-
What does the after-sales service for Mortenon M1 include?We provide comprehensive after-sales services for the Mortenon M1, including equipment installation and commissioning, operator training, regular maintenance, troubleshooting, and remote technical support. Additionally, spare parts are supplied promptly to ensure stable and worry-free operation. Our service team responds quickly, and users can contact us anytime via hotline, email, or other channels. We will arrange timely support based on the situation to ensure the equipment runs efficiently and reliably.